How to Choose the Right Medical Cart for Hospitals
In modern hospitals, efficiency directly affects patient care. Medical carts are no longer simple transport tools—they function as mobile nursing stations, emergency support units, and workflow assistants used throughout the day. Choosing the right medical cart helps improve staff efficiency, reduce physical strain, and support infection control standards.
This guide outlines the key factors hospitals should consider when selecting medical carts, focusing on materials, design features, and supplier capabilities.
Medical Cart Materials: Why Lightweight Aluminum Matters
Traditionally, stainless steel has been widely used for medical carts due to its strength and durability. However, daily hospital operations require staff to push carts over long distances, making weight an important consideration—especially for carts used frequently, such as mobile medical medication cart systems in wards and pharmacies.
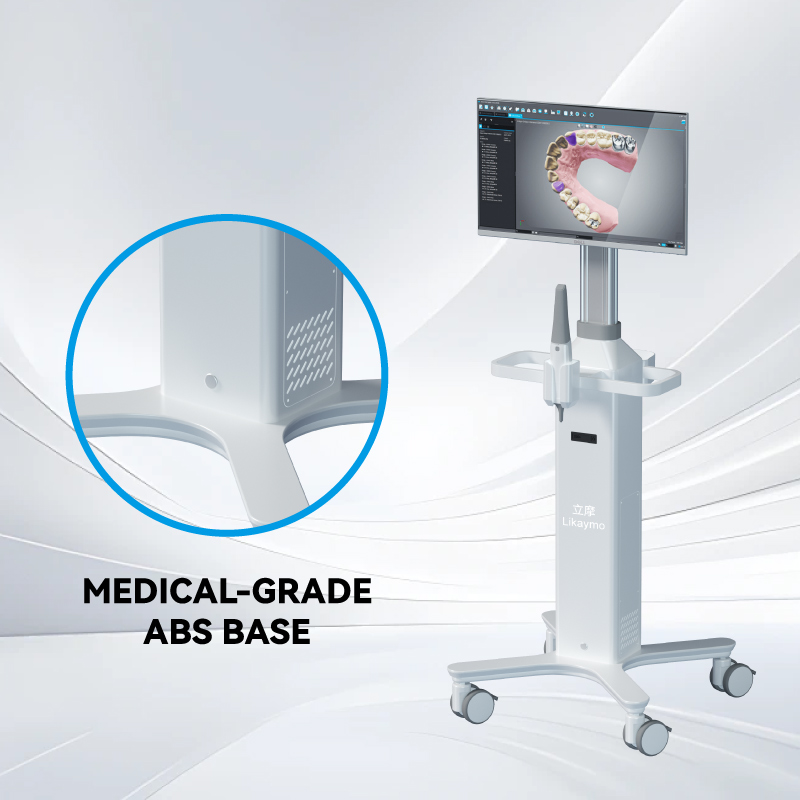
Aerospace-grade aluminum alloy is increasingly preferred because it is significantly lighter while still offering excellent load-bearing capacity and impact resistance. This reduces physical strain on medical staff and improves maneuverability in busy corridors. In addition, aluminum naturally resists corrosion and rust, making cleaning and disinfection easier—an essential factor for maintaining infection control standards. Its smooth surface and modern appearance also contribute to a cleaner, more professional hospital environment.

Medical Cart Design Features That Improve Daily Use
Beyond materials, practical design details determine how well a medical cart performs in real hospital scenarios.
High-quality wheels should move smoothly and quietly, especially in wards where minimizing noise is important for patient comfort. Reliable braking systems are equally critical, allowing carts to remain stable during procedures or urgent situations, such as when using a medical emergency cart in fast-paced clinical environments.
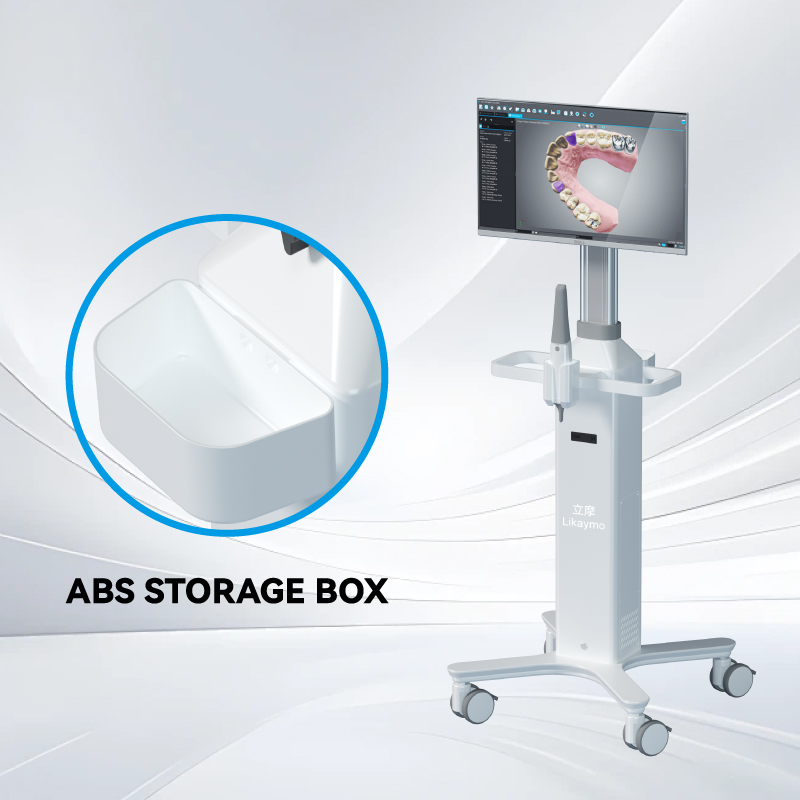
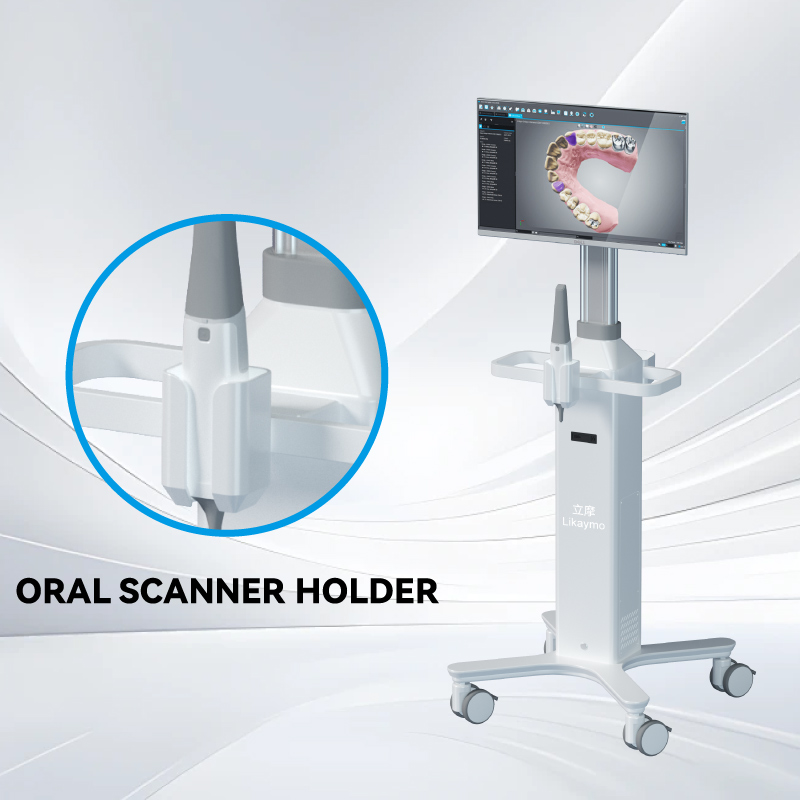
Adjustable handle heights help accommodate staff of different statures, reducing fatigue during long shifts. Modular design is another key factor. Carts that allow drawers, baskets, and accessory holders to be added or removed can be adapted to the needs of different departments, improving usability and extending product lifespan.

Choosing a Medical Cart Supplier: Brand, Service, and Customization
Selecting a medical cart is not just about the product itself, but also about the supplier’s understanding of clinical workflows. Hospitals increasingly rely on solutions like a mobile clinical workstation, which combines storage, mobility, and digital support into a single platform.
Manufacturers that focus on ergonomic design, lightweight structures, and modular configurations are better positioned to meet these evolving needs. Some suppliers, such as Likaymo, emphasize aluminum-based structures combined with customizable layouts, allowing carts to be adapted for emergency care, anesthesia, or routine nursing tasks. The ability to provide customization and long-term support ensures that carts integrate smoothly into daily operations rather than becoming fixed, one-size-fits-all tools.
Matching Medical Carts to Different Hospital Departments
Different departments often prioritize different cart features:
l Emergency departments: high mobility, stable braking systems, modular storage
l Nursing stations: ergonomic handles, quiet wheels, organized drawers
l Procedure and anesthesia rooms: easy-to-clean surfaces and customized configurations
Choosing carts based on real usage scenarios helps maximize efficiency and safety.
Focus on Practical Needs and Long-Term Value
Selecting medical carts is a long-term investment in workflow efficiency, staff well-being, and patient safety. Rather than focusing solely on price or traditional materials, hospitals should consider lightweight construction, user-centered design, and the supplier’s ability to adapt carts to specific clinical environments.
By choosing medical carts based on actual operational needs, hospitals can build more efficient, flexible, and sustainable care systems.If you would like to learn more, please visit our website:www.likaymo.com